
JACKSONVILLE, Florida – May 16, 2018 – TapImmune Inc. (NASDAQ: TPIV), a clinical-stage immuno-oncology company, today announced that it has assembled a Scientific Advisory Board (SAB) composed of leading experts in the field of immuno-oncology and cell therapy and that will become effective in conjunction with the proposed merger between the Company and Marker Therapeutics, Inc. The goal of the SAB will be to support the continued clinical development of the Company’s transformative, non-genetically engineered, multi-antigen T cell therapy platform. Foundational members of the SAB include senior medical scientists from the Center for Cell and Gene Therapy at Baylor College of Medicine, including its founding Director and former President of the American Society for Gene and Cell Therapy (ASGCT) and of the International Society for Cell Therapy, Malcolm K. Brenner, M.D., Ph.D. Joining Dr. Brenner are colleagues Helen E. Heslop, M.D., D.Sc. (Hon), the current president of ASGCT, and Cliona M. Rooney, Ph.D., who collectively laid the foundation and were the first to validate the use of antigen-specific T cell transfer to prevent and cure viral malignancies in stem cell transplant patients.
Peter Hoang, President and CEO of TapImmune, stated, “I am absolutely delighted and humbled to have the support and commitment of world leaders in the advancement of immunotherapy such as Malcolm Brenner, Cliona Rooney and Helen Heslop to help us advance our platform. Dr. Brenner is a household name within the CAR-T and TCR field for his pioneering work in advancing T cell therapies. As the founding Director of the Center for Cell and Gene Therapy, he created one of the world’s foremost institutions in the field of T cell therapies, and he is among the field’s most prominent and well-regarded thought leaders based on his scientific and translational studies.
“Dr. Heslop is the current President of the American Society for Gene and Cell Therapy and a key investigator in the translation of the science of immunology from the laboratory into the clinic. She is one of the field’s best-known experts in developing methods and procedures for generating cytotoxic T cell lymphocytes that can be used to drive life-changing therapeutic results for patients. Dr. Rooney is a global leader in the field of T cell-based therapy, authoring many seminal studies in virus and non-virus-associated malignancies. Her work has helped change the lives of hundreds of patients while meaningfully advancing the science of cancer treatment.
“We’re proud to attract such world-leading advisors as we seek to transform the field of cell therapy for cancer. Our highly differentiated platform, which has generated potentially groundbreaking efficacy and safety results in early clinical trials, originated with this team at Baylor College of Medicine. I have tremendous respect for Drs. Brenner, Heslop, and Rooney and I’m honored to be able to access their unmatched expertise and experience, helping to ensure that the value our T cell therapies can deliver for patients is effectively validated in future clinical studies. We look forward to moving ahead with their support as the proposed merger is completed.”
SAB Member Bios:
Malcolm K. Brenner, M.D., Ph.D.
Dr. Brenner is the founding director of the Center for Cell and Gene Therapy and the Fayez Sarofim Distinguished Service Professor at Baylor College of Medicine in the Departments of Medicine, Pediatrics, and Human and Molecular Genetics. He is also a member of the Texas Children’s Cancer and Hematology Center, the Stem Cell and Regenerative Medicine Center, and the Dan L. Duncan Comprehensive Cancer Center at Baylor. Over the past 30 years, Dr. Brenner has devoted his career as a physician-scientist to the field of stem cell transplantation through the therapeutic use of T cell immunologic approaches and genetic engineering strategies. He served as Editor-in-Chief of Molecular Therapy and as former President of the American Society for Gene and Cell Therapy (ASGCT) and International Society for Cell Therapy. Dr. Brenner has earned widespread recognition for his scientific achievements and leadership in the field, including the ASGCT Outstanding Achievement Award, Human Gene Therapy’s Pioneer Award, and the American Society of Hematology Mentor Award. Most recently Dr. Brenner has been elected to the prestigious National Academy of Medicine for his contributions. He obtained his BA and medical degrees as well as his Ph.D. from the University of Cambridge in the UK where he became a fellow of the Royal College of Pathologists and the Royal College of Physicians. Dr. Brenner is a co-founder of ViraCyte, LLC.
Helen E. Heslop, M.D., D.Sc. (Hon)
Dr. Heslop is Professor of Medicine and Pediatrics and Director of the Center for Cell and Gene Therapy at Baylor College of Medicine, Houston Methodist Hospital, and Texas Children’s Hospital. She is the Dan L. Duncan Chair and the Associate Director of Clinical Research at the Dan L. Duncan Cancer Center. Trained as a physician-scientist, Dr. Heslop is a prominent figure engaged in translational research focusing on adoptive T cell immunotherapy to improve hematopoietic stem cell transplantation and cancer therapy. In collaboration with Drs. Brenner and Rooney, her initial studies were the first to demonstrate the feasibility of using EBV-specific T cells to prevent and treat EBV-associated malignancy in stem cell transplant patients, thereby validating the safety and efficacy of adoptive T cell transfer as a therapeutic modality. Dr. Heslop was a Doris Duke Distinguished Clinical Research Scientist and serves as the current President elect of the American Society for Gene and Cell Therapy (ASGCT) and a past President of the Foundation for Accreditation of Cell Therapy (FACT) and the American Society of Blood and Marrow Transplantation. She received her M.B.ChB, M.D. and D.Sc. (Hon) in Hematology from the University of Otago in New Zealand. Dr. Heslop is a co-founder of ViraCyte, LLC.
Cliona M. Rooney, Ph.D.
Dr. Rooney is a Professor in the Departments of Pediatrics, Molecular Virology and Microbiology, and Immunology and Director of Translational Research Laboratories at the Center for Cell and Gene Therapy at Baylor College of Medicine. Dr. Rooney is renowned virologist and immunologist who, in collaboration with Drs. Heslop and Brenner, was the first to demonstrate that antigen-specific T cells generated in the laboratory could prevent and cure viral-associated malignancies in humans following hematopoietic stem cell transplantation. She has been key in extending this successful strategy to develop and clinically test a range of post-transplant viral infections and diseases. Dr. Rooney serves on the Editorial Boards for several scientific journals, including Molecular Therapy, Cytotherapy, Molecular and Cellular Therapies. Dr. Rooney holds a Ph.D. in immunology from the University of Cambridge and a B.Sc. in Genetics from the University of East Anglia in England. Dr. Rooney is a co-founder of ViraCyte, LLC.
Transaction Adds Multi-Antigen Targeted Cell Therapy Platform to
TapImmune’s Peptide Vaccine Portfolio
TapImmune Raises $5.1 million in Financing from Current Stockholders
TapImmune will Finalize a Strategic Alliance with Baylor College of Medicine
Conference Call and Live Audio Webcast Scheduled Today at 8:00 a.m. ET
JACKSONVILLE, Florida – May 15, 2018 – TapImmune Inc. (NASDAQ: TPIV) (“TapImmune”) today announced that it has entered into a definitive merger agreement to acquire Marker Therapeutics, Inc. (“Marker”), a privately-held clinical-stage developer of a transformative, non-genetically engineered, multi-antigen T cell therapy platform. The proposed transaction will be a merger-of-equals under which the stockholders of TapImmune and Marker will each own approximately 50% of the combined company, prior to any issuances of additional shares in a contemplated financing. The proposed merger remains subject to certain conditions, including that financing and the approval of TapImmune stockholders. TapImmune and Marker will host a conference call and webcast today at 8:00 a.m. ET.
Peter Hoang, President and CEO of TapImmune, stated, “I believe that the new therapies we are acquiring with Marker in this transaction represent the next major leap forward in cell therapy for cancer. The merger adds to our product pipeline a synergistic portfolio of highly-differentiated T cell therapies that has demonstrated potentially groundbreaking results in early clinical trials in lymphoma, acute myeloid leukemia (AML), and multiple myeloma.”
“With this merger, I believe we have the opportunity to significantly disrupt the CAR-T and TCR field,” added Mr. Hoang. “Compared to current gene-modified T cell therapies such as CAR-T and TCR, the therapies we are acquiring in this transaction are:
Highly efficacious and extremely durable, without the need for lymphodepletion before infusion: In our Phase I lymphoma study, we saw complete responses (CRs) in 50-60% of our evaluable patients, a rate comparable to the best reported CAR-T studies in lymphoma. However, unlike in CAR-T studies, we have yet to see a single disease relapse in any responder, whereas typically 30% or more of patients with CR in today’s CAR-T studies relapse within one year. In fact, more than half of our CRs are in durable remission beyond a year, with several patients being relapse-free beyond 2-3 years.
Non gene-modified: Unlike current CAR-T and TCR approaches, our cell therapeutic approach requires no genetic modification of T cells, which will allow us to manufacture the product at a fraction of the cost, with substantially reduced complexity of manufacturing.
Significantly less toxic than CAR-T: With more than 60 patients treated, our therapies have never caused cytokine release syndrome (CRS) or a related serious adverse event (SAE) in patients treated with our therapy. In fact, we have seen only one grade III adverse reaction that was considered possibly related in our patients to date, versus a 95% incidence rate of grade III or higher adverse events in recent CAR-T studies.
Multi-antigen specific: Our new technology identifies and selects for substantially all T cells that are specific to any peptide epitope of the antigens we target, including very rare clones that are otherwise undetectable in our deep gene sequencing of patients’ peripheral blood. Compared to current CAR-T and TCR approaches, which target a single epitope of only one target antigen, our multi-specific T cell therapy products that are currently in clinical trials have been shown to consist of approximately 4,000 unique T cell clonotypes targeting up to five different tumor-associated antigens.
Capable of driving an endogenous immune response: We see consistent evidence of “epitope spreading” in our patients, meaning that our therapy is inducing the patient’s native T cells (that are specific to tumor associated antigens that are not targeted by our infused product) to expand and contribute to a lasting anti-tumor effect. This phenomenon, also known as “antigen spreading,” has been the stated goal of many CAR-T and TCR developers, but we believe that our therapy is the first to show a consistent ability to drive this effect.
Capable of addressing patients currently inaccessible to CAR-T therapies: Because our product is derived from natural patient T cells without gene modification, we are able to treat patients earlier and in indications that are currently not addressable by CAR-T therapies. We have seen strong patient responses that are highly durable, while seeing no associated graft versus host disease (GvHD) in post-transplant relapsed/refractory AML, a setting where currently the only available alternative therapy is a donor lymphocyte infusion (DLI). DLIs generally have very low patient response rates with high rates of severe associated GvHD. CAR-T approaches cannot currently be used in post-transplant relapsed/refractory AML because most envisioned CAR-T therapies are targeted to antigens expressed on hematopoietic stem cells, potentially causing fatal neutropenia. Furthermore, our therapies can be used as maintenance therapies and in earlier treatment settings than can CAR-T or TCR approaches, which would generate significant toxicities in those settings.”
“Executing this strategic merger with Marker Therapeutics will be fundamentally transformational for TapImmune, enriching our strong immuno-oncology pipeline with a revolutionary multi-antigen targeted cell therapy platform. We believe this technology will be a game-changer for the cell therapy industry, potentially overcoming the well-known limitations of today’s CAR-T and TCR approaches,” concluded Mr. Hoang. “Combined with the four ongoing Phase 2 clinical trials in the TapImmune platform, I believe we are creating a best-in-class cancer immunotherapy platform. With Marker’s peptide-based cell therapy platform, we believe that there is an excellent fit with TapImmune’s extensive experience and expertise in the research, development, manufacturing and manipulation of peptide-based immunotherapies. Furthermore, the integration of the two companies provides us with a compelling opportunity to create a unique and highly differentiated company in the immuno-oncology field.”
John Wilson, CEO of Marker, said, “I feel very fortunate to have been entrusted with one of the premier programs of Baylor College of Medicine’s Center for Cell and Gene Therapy, and to integrate it with TapImmune to provide this exceptional technology with a strong commercial pathway. We have great respect for the work that the TapImmune team has done within the immuno-oncology field and believe integrating our respective peptide-based technologies will drive significant advances in the field. By combining TapImmune’s experience and expertise in multi-epitope peptide-based approaches to T cell activation with Marker’s multi-targeted T cell therapy, while simultaneously leveraging the know-how and facilities of Baylor College of Medicine’s Center for Cell and Gene Therapy, we intend to chart a groundbreaking course toward more effective, less complex, non-toxic and cost-effective cancer treatments. Our respective development teams are eager to join forces and drive a unique product pipeline to patients in need. We believe the merger will accelerate clinical development, particularly for the cell therapy platform, which has generated encouraging patient responses in our clinical trials to date. I look forward to taking a position on the post-merger Board of Directors where I can leverage my T cell manufacturing expertise and help the Company implement a highly practical and economical manufacturing platform. By avoiding the need for genetic engineering, the manufacturing process can be greatly simplified, providing us with a great opportunity to successfully address the cost issues that currently plague the field.”
In conjunction with the transaction, TapImmune intends to finalize a strategic alliance with Baylor College of Medicine which will include sponsored research, manufacturing support, and advancing early stage clinical trials at the institution.
Merger Related Financing
TapImmune is currently in discussions with a syndicate of leading healthcare-focused institutional investors with respect to a potential financing in conjunction with the merger that will be expected to fund the combined company into 2020.
Private Placement of Common Stock, Warrant Exercises and Financing Commitment
In support of TapImmune’s initiatives, including the merger, the Company has entered into agreements with certain institutional stockholder and warrant holders that are expected to provide the Company with approximately $5.1 million in equity financing. The Company’s largest stockholder, Eastern Capital Limited, has entered into a Common Stock Purchase Agreement with the Company pursuant to which it will purchase 1.3 million shares of common stock at a price per share of $2.40 providing gross proceeds to the Company of approximately $3.1 million. Other selected institutional holders of outstanding warrants have entered into warrant amendment agreements with the Company to exercise their warrants at an exercise price of $2.50 per share. Upon closing of the warrant amendment agreements, such participating institutional holders will exercise approximately 783,000 warrants providing aggregate proceeds to the Company of approximately $2.0 million.
In addition, Mr. John Wilson, CEO of Marker, has provided a written commitment for additional financing to the Company of up to $1.0 million.
About the Proposed Merger
Existing Marker stockholders will receive newly issued shares and warrants of TapImmune common stock in connection with the proposed merger equal to the number of shares and warrants of TapImmune outstanding at the closing of the merger. TapImmune currently has 10.7 million shares of common stock and approximately 7.0 million warrants and options outstanding (excluding any shares issuable in connection with the financing referenced above). The number of warrants issuable to Marker are subject to increase based upon certain conditions related to the terms of any additional financing closed concurrently with the merger. On a pro forma basis for the combined company, current TapImmune stockholders and current Marker stockholders are each expected to own approximately 50% of the combined company, prior to the contemplated issuance of shares in the financing that is expected to occur concurrently with the merger.
The transaction has been unanimously approved by the board of directors of both companies. The proposed merger is expected to close in the second half of 2018, subject to completion of the concurrent financing and the approval of the stockholders of each company as well as other customary conditions. The merger agreement contains further details with respect to the proposed merger.
Nomura Securities International, Inc. acted as the exclusive financial advisor to TapImmune. Seyfarth Shaw LLP served as legal counsel to TapImmune. Winthrop & Weinstine, PA served as legal counsel to Marker.
Management and Organization
Following the closing of the proposed merger, TapImmune CEO Peter Hoang will be President and CEO of the combined company. Marker CEO John Wilson will join the combined company’s Board of Directors as will Juan Vera, M.D., a Co-Founder of Marker. The board of directors of the combined company is expected to consist of eight members, three of whom will be designated by TapImmune, three of whom will be designated by Marker, and two of whom will be designated by the investor syndicate. In addition, Marker Co-Founder Ann Leen, Ph.D. will be appointed to the new position of Chief Scientific Officer. Michael Loiacono will continue to serve as Chief Financial Officer and Richard Kenney, M.D. will continue as Acting Chief Medical Officer. Additionally, TapImmune is expected to announce the formation of a new Scientific Advisory Board which will become effective on the closing date of the merger.
Additional Information about the Proposed Merger
In connection with the proposed merger, TapImmune intends to file relevant materials with the Securities and Exchange Commission, or the SEC, including a proxy statement. Investors and security holders of TapImmune are urged to read these materials when they become available because they will contain important information about TapImmune, Marker and the proposed merger. The proxy statement and other relevant materials (when they become available), and any other documents filed by TapImmune with the SEC, may be obtained free of charge at the SEC web site at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by TapImmune by directing a written request to: TapImmune Inc., 5 West Forsyth Street, Suite 200, Jacksonville, FL 32202, Attn: Investor Relations. Investors and security holders are urged to read the proxy statement and the other relevant materials when they become available before making any voting decision with respect to the proposed merger.
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities in connection with the proposed merger shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
Participants in the Solicitation
TapImmune and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of TapImmune in connection with the proposed transaction. Information regarding the special interests of these directors and executive officers in the proposed merger will be included in the proxy statement referred to above. Additional information regarding the directors and executive officers of TapImmune is also included in its Annual Report on Form 10-K for the year ended December 31, 2017, filed with the Securities and Exchange Commission (the “SEC”) on March 23, 2018. This document is available free of charge at the SEC’s web site (www.sec.gov) and from Investor Relations at TapImmune at the address described above.
Conference Call and Webcast Information
The companies will host a conference call and live audio webcast today, May 15, 2018, at 8:00 a.m. ET. Interested participants and investors may access the conference call by dialing:
1 (855) 238-2333 (U.S.) or
1 (412) 317-5215 (International)
To access the live audio webcast, visit the Events section of the TapImmune website https://markertherapeutics.com/events/. The webcast will be archived for 90 days beginning at approximately 10:30 a.m. ET, on May 15, 2018.
Live Audio Webcast Scheduled for May 17th at 8:00 a.m. CT
JACKSONVILLE, Florida – May 15, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company, today announced that it will host a breakfast meeting for analysts and investors this Thursday, May 17, 2018, at the Blackstone Hotel in Chicago. During the meeting, experts will discuss the incorporation of Marker Therapeutics’ groundbreaking multi-antigen T cell therapy technology into TapImmune’s existing cancer immunotherapy pipeline, pending closing of the proposed merger of the two companies announced earlier today. Attendees will discuss the technology platform with Marker Co-Founders Juan Vera, M.D. and Ann Leen, Ph.D., and renowned cell therapy thought leader Malcolm Brenner, M.D., Ph.D. The group will also participate in Q&A along with TapImmune management.
Meeting Details
Date: Thursday, May 17, 2018
Time: 7:30am – 10:00am CT
Location: The Blackstone Hotel, 636 South Michigan Avenue, Chicago, IL 60605
To access the live audio webcast of the meeting and accompanying slides, visit the Events section of the TapImmune website https://markertherapeutics.com/events/. The webcast will be archived for 30 days beginning at approximately 10:30 a.m. ET, on May 17, 2018.
JACKSONVILLE, Florida, May 7, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that its President and CEO, Peter Hoang, will give a company presentation at the 2018 NYC Oncology Investor Conference, held May 8-9, 2018 at Wilson Sonsini Goodrich & Rosati in New York City.
Presentation Details
Date: Wednesday, May 9th, 2018
Time: 3:00 PM ET
JACKSONVILLE, Florida, May 1, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that its President and CEO, Peter Hoang, will participate in a panel discussion and give a company presentation at the 2018 Disruptive Growth & Healthcare Conference, held May 8-9, 2018 at Reed Smith LLP in New York City.
Disruptive Growth & Healthcare Conference
Disruptive ImmunoTherapies Panel
Date: Wednesday, May 9th, 2018
Time: 9:30 AM ET
Location: Track 2 | Room C/D
Webcast: https://www.webcaster4.com/Webcast/Page/1861/25809
TapImmune Company Presentation
Date: Wednesday, May 9th, 2018
Time: 11:35 AM ET
Location: Track 1 | Room A/B
Recent Corporate and Clinical Developments:
- Published long-term immune response and progression-free survival data from completed Phase 1 clinical study of TPIV200
- Commenced dosing in 280-patient, grant-funded Phase 2 study of TPIV200 in women with advanced TNBC
- Enhanced IP portfolio for PolyStart™ technology, expanding to cover any polypeptide sequences comprising poly-antigen arrays (PAAs)
- Appointed Dr. Richard Kenney as Acting Chief Medical Officer
Upcoming Anticipated Milestones:
- Q3 2018: Report interim immune response data from ongoing Phase 2 TNBC
- 2H 2018: Report interim results from ongoing Phase 2 study of TPIV200 in combination with AstraZeneca’s durvalumab in patients with platinum-resistant ovarian cancer
- 2018: Mayo Clinic to initiate Phase 1b/2a study of TPIV100 in women with HER2/neu+ ductal carcinoma in situ (DCIS) breast cancer
- 1Q 2019: Report interim safety and futility results from Phase 2 ovarian cancer study
JACKSONVILLE, FLORIDA – April 5, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today provided its business update for the fourth quarter and year-end 2017. A public conference call and live audio webcast is scheduled for today at 4:30 p.m. ET.
“Throughout 2017, we made significant advances toward achieving our goals and reaching our milestones,” said Peter Hoang, President and CEO of TapImmune. “We recently announced the publication of new clinical data for our multi-epitope T-cell vaccine targeting folate receptor alpha, TPIV200, in patients with ovarian and breast cancer. In this publication we showed an encouraging potential progression-free survival benefit in women with ovarian cancer in their first remission, which we are currently exploring further in an ongoing randomized Phase 2 study. Should we see a similar, prolonged PFS in this larger study, we believe that TPIV200 could have a viable pathway toward potential approval in this indication, for which it has FDA Fast Track designation. We remain on track to conduct an interim safety and futility analysis for the Phase 2 study by mid-2019.”
Mr. Hoang continued, “With multiple Phase 2 and Phase 1/2 clinical studies ongoing, several of which are funded by U.S. Department of Defense grants, and our preclinical PolyStart™ technology maturing rapidly to the point where it may drive value through strategic partnership, we believe TapImmune is on a strong growth trajectory that will continue through 2018 and beyond. Our progress will be measured by continued milestone execution and we look forward to building value at each step along the way.”
Current Clinical Studies:
TPIV200: Lead T-cell vaccine targeting folate receptor alpha
- FDA Fast-tracked Phase 2 maintenance therapy study in platinum-sensitive ovarian cancer
TapImmune is currently enrolling women who have completed initial therapy with a platinum regimen and are in first remission. Enrollment remains on track with projections and the company plans to conduct a blinded interim safety and futility analysis once the data from the first half of enrollment is achieved and responses mature, which is currently expected by mid-2019. This program benefits from FDA Fast Track and Orphan Drug designation. - Multi-center Phase 2 dosing study in triple-negative breast cancer
The randomized study is designed to determine the optimal vaccine dose and regimen that may maximize the anti-tumor immune response in maintenance-phase patients who have completed standard surgery and chemotherapy/radiation. Enrollment in this study is complete and TapImmune expects to report interim immune response data in the third quarter 2018. - U.S. Department of Defense (DoD)-funded Phase 2 efficacy study in advanced triple-negative breast cancer
In late 2017, the Mayo Clinic successfully dosed the first patient in a Phase 2 study designed to evaluate the safety and efficacy of TPIV200 in prolonging disease-free survival in women with advanced triple-negative breast cancer. This 280-patient randomized, double-blind and placebo-controlled study is completely funded by a $13.3 million grant from the U.S. DoD. - Memorial Sloan Kettering-sponsored Phase 2 combination study with AstraZeneca’s durvalumab in platinum-resistant ovarian cancer
Data from the first 27 patients enrolled in the study are currently being analyzed by the study’s clinical investigators at MSKCC. TapImmune anticipates reporting the results based on these 27 patients once patient analysis at MSKCC is released.
Planned Clinical Studies:
TPIV100/110 T-cell vaccine targeting HER2/neu:
- Mayo Clinic is expected to initiate a Phase 1b/2a study of TPIV100 in women with an early form of breast cancer called ductal carcinoma in situ (DCIS). This study is also fully funded by a grant from the U.S. DoD. If successful, TapImmune’s HER2/neu-targeted vaccine may complement standard surgery and chemotherapy.
- TapImmune planned to submit FDA filings for its five-peptide HER2 vaccine, TPIV110, and begin a Phase 1/2 clinical study in women with HER2-low breast cancer. In the fourth quarter, the U.S. DoD expressed interest in fully funding a larger Phase 2 clinical study using TPIV110 in combination with Herceptin® (trastuzumab) in HER2neu+ breast cancer. TapImmune is currently engaged in discussions with the Mayo Clinic and the U.S. DoD regarding this Phase 2 study, which would supplant the previously planned TapImmune-sponsored Phase 1/2 study. TapImmune will provide an update once the details of the study and the required FDA filings are finalized.
Conference Call and Webcast Information:
To access the live conference call on April 5, 2018, at 4:30pm ET you may use:
- (855) 238-2333 (U.S.)
- (412) 317-5215 (International)
To access the live audio webcast, visit the Events section of the TapImmune website https://markertherapeutics.com/events. The webcast will also be archived for 90 days beginning at approximately 6:30 p.m. ET, on April 5, 2018.
TAPIMMUNE INC.
CONSOLIDATED BALANCE SHEETS

TAPIMMUNE INC.
CONSOLIDATED STATEMENT OF OPERATIONS
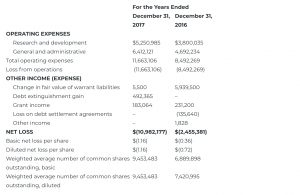
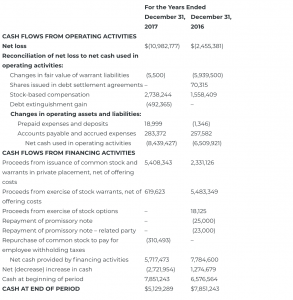
TAPIMMUNE INC.
CONSOLIDATED STATEMENT OF CASH FLOWS
(Accesswire — March 28, 2018) – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that CEO Peter Hoang will present at The MicroCap Conference, to be held April 9-10, 2018, at The Essex House in New York City.
The MicroCap Conference
TapImmune Presentation
Date: Monday, April 9, 2018
Time: 9 a.m. (Eastern Time)
Location: The Essex House, Track 1
An audio webcast will be accessible via the News and Events section of the TapImmune website: https://markertherapeutics.com/events. An archive of the audio will remain available for 90 days following the presentation.
CONFERENCE CALL AND LIVE AUDIO WEBCAST SCHEDULED FOR TODAY AT 9:00 AM ET TO DISCUSS THE RESULTS
Jacksonville FL, March 15, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced the publication of clinical data from a Phase 1 trial of TPIV200, the company’s multi-epitope T-cell vaccine targeting Folate Receptor Alpha (FRa) in patients with ovarian and breast cancer. The study by Kalli, Block, et al., titled, “Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients”, will be published in the leading peer-reviewed oncology journal Clinical Cancer Research and will be available online today. The results show that TPIV200 vaccination was well tolerated by all patients and over 90% developed robust and durable antigen-specific immune responses against FRa without regard for HLA type, which aligns with the intended mechanism of action of the vaccine.
The Phase 1 study was not designed to show efficacy and involved a small number of patients. Yet, a retrospective analysis showed that among the subset of ovarian cancer patients who were vaccinated following a first remission (n=10), median progression-free survival (PFS) was extended compared to published clinical results1 for a similar patient population that received standard of care chemotherapy. TapImmune has published a white paper that further details this preliminary data and its potential implications for the company’s ongoing Phase 2 clinical trial of TPIV200 in women with platinum-sensitive ovarian cancer.
Peter Hoang, President and CEO of TapImmune, stated, “Although this safety trial was not designed to evaluate clinical efficacy outcomes, all patients remained alive at last follow-up, at least two years following initiation of immunization, and the large majority of vaccinated patients developed lasting immune responses against multiple FRa epitopes. The potential PFS benefit observed in women with ovarian cancer in first remission is very intriguing to TapImmune and our clinical investigators, especially in the context of our ongoing Phase 2 study of TPIV200, which is enrolling patients at the same stage of disease. While our population of 10 patients in ovarian first remission cannot be deemed to be statistically significant, the observed median PFS of 528 days is certainly an interesting result, particularly in light of the historical data where patients receiving stand-of-care in this setting had a median PFS in the 313-day range. Should we see a similar result in our larger ongoing randomized, double-blind, controlled Phase 2 trial, we believe that TPIV200 has a viable pathway toward potential approval in this indication. Enrollment in the study is currently ongoing, and we expect to conduct an interim analysis by mid-2019, once the data from the first half of enrollment is achieved.”
Dr. Richard Kenney, Acting Chief Medical Officer for TapImmune, stated, “There remains a significant unmet need in ovarian cancer to delay or prevent disease recurrence following successful platinum-based therapy. That is why TapImmune is focusing on TPIV200 immunotherapy at this earlier stage in the treatment paradigm where no approved therapies currently exist in the ongoing Phase 2 study. Women in first remission are likely to have healthier immune systems, having not gone through multiple rounds of chemotherapy and cancer recurrence, and may be better positioned to benefit from vaccination with TPIV200. We are grateful to Dr. Keith Knutson and his team for conducting the study and publishing the results. Should TPIV200 demonstrate a significant benefit in prolonging disease-free survival in this patient population, TapImmune will be well positioned to address a unique segment within the ovarian cancer treatment landscape.”
Conference Call and Webcast Information:
The company will host a conference call and live audio webcast today, March 15, 2018, at 9:00 AM ET. Interested participants and investors may access the conference call by dialing either:
- 1 (855) 238-2333 (U.S.)
- 1 (412) 317-5215 (International)
To access the live audio webcast, visit the Events section of the TapImmune website https://markertherapeutics.com/events/. The webcast will also be archived for 90 days beginning at approximately 10:30 a.m. ET, on March 15, 2018.
1 PERREN, ET AL., (2011) “A PHASE 3 TRIAL OF BEVACIZUMAB IN OVARIAN CANCER.” NEJM 365:2484-96
JACKSONVILLE, Florida, March 14, 2018 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that its President and CEO, Peter Hoang, will participate in a panel discussion and give a company presentation at the Sachs BioCapital USA Forum, held March 21st, 2018. He will also give a company presentation and live webcast at the 26th Annual Wall Street Investor Forum, held March 22nd, 2018. Both conferences will take place in New York City.
Sachs BioCapital USA Forum
Future of IO: Technologies & Market Opportunities Panel
Date: Wednesday, March 21, 2018
Time: 10:15 AM (Eastern Time)
Location: New York Academy of Sciences, Plenary Room
TapImmune Company Presentation
Date: Wednesday, March 21, 2018
Time: 3:00 PM (Eastern Time)
Location: The New York Academy of Sciences, Plenary Room (Presenting Track B)
26th Annual Wall Street Investor Forum
TapImmune Company Presentation
Date: Thursday, March 22, 2018
Time: 11:05 AM (Eastern Time)
Location: The University Club of NYC, 9th Floor (Track 1)
The audio webcast of the 26th Annual Wall Street Investor Forum will be accessible via the News and Events section of the TapImmune website: https://markertherapeutics.com/events. An archive of the audio will remain available for 90 days following the presentation.
JACKSONVILLE, Florida, February 6, 2018 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that its President and CEO, Peter Hoang, will present at the 2018 BIO CEO & Investor Conference, held February 12-13, 2018, in New York City. He will also attend the 11th Annual European Life Sciences CEO Forum & Exhibition, held February 26-27, 2018, in Zurich, Switzerland.
TapImmune Presentation Details:
Event: 2018 BIO CEO & Investor Conference
Date: Monday, February 12, 2018
Time: 2:30 PM (Eastern Time)
Location: New York Marriott Marquis, Odets Room
An audio webcast will be accessible via the News and Events section of the TapImmune website: https://markertherapeutics.com/events. An archive of the audio will remain available for 90 days following the presentation.