
*UPDATE*
As a result of a leg injury suffered over the holidays, TapImmune President & CEO Peter Hoang has been compelled to cancel his planned presentation at Biotech Showcase in San Francisco. While fixing his son’s ceiling vent, Mr. Hoang slipped and fell, resulting in a moderate tear of his calf muscle, an injury that has made him unable to travel this week. On the advice of his physicians, he will use the week of January 8 to recuperate but will plan to return to his travel duties the week of January 15.
JACKSONVILLE, Florida, January 3, 2018 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that its President and CEO, Peter Hoang, will present at Biotech Showcase™ 2018, held January 8-10, 2018, in San Francisco, California.
TapImmune Presentation Details:
Date: Tuesday, January 9, 2018
Time: 4:30 p.m. (Pacific Time)
Location: Hilton Hotel, Franciscan A Ballroom; San Francisco, CA[
JACKSONVILLE, Florida, December 12, 2017 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that the first patient has been enrolled in a Phase 2 randomized, multi-center, double-blinded, placebo-controlled clinical trial of TapImmune’s novel therapeutic vaccine candidate TPIV200. The 280-patient trial, sponsored by Mayo Clinic, received $13.3 million in grant funding from the U.S. Department of Defense (DoD) to evaluate the prevention of cancer recurrence in women with triple-negative breast cancer (TNBC) who have completed first-line surgery and radiotherapy/chemotherapy.
TPIV200 is a novel, multi-epitope, peptide-based cancer vaccine that has been shown to induce a robust and long-lasting “memory” T-cell immune response directed against folate receptor alpha (FRa), a molecule that is overexpressed on the surface of the vast majority of TNBC cancer cells and is associated with cancer recurrence. As an off-the-shelf vaccine consisting of several carefully chosen FRa peptides, TPIV200 is uniquely able to stimulate both T “helper” cells and T “killer” cells to target tumor cells and is expected to cover greater than 85% of human genotypes worldwide.
“We remain grateful to the U.S. Department of Defense and Mayo Clinic for enabling TapImmune to gain invaluable clinical safety and efficacy insight for TPIV200 under this grant,” said TapImmune President and CEO Peter Hoang. “We believe TPIV200 and our other vaccine candidates have an important role to play within the current immuno-oncology ecosystem by potentially bridging a critical gap not currently addressed by other immunotherapies, which have shown promise in only a small number of patients. Unlike current approaches, TapImmune’s vaccines are designed to produce broad-based, durable T-cell responses in the vast majority of patients, which we believe are essential for improving clinical outcomes and ensuring potential regulatory and commercial success. We look forward to providing updates appropriately as this exciting Phase 2 study continues to enroll patients.”
TapImmune and its clinical partners are evaluating TPIV200 in multiple ongoing Phase 2 trials for treating ovarian and breast cancer, including a randomized dosing trial in TNBC that recently completed patient enrollment. The four-arm trial is designed to help determine the optimal TPIV200 vaccine dose and regimen to maximize patients’ anti-tumor immune responses. Interim immunogenicity results from this ongoing study are anticipated in the first half of 2018.
Keith L. Knutson. Ph.D., Professor of Immunology in the Department of Immunology, and Edith A. Perez, M.D., Professor of Medicine in the Division of Hematology and Oncology, both at Mayo Clinic’s Florida campus in Jacksonville, Florida, are the recipients of the U.S. Department of Defense grant and are leading the Phase 2 trial.
Mayo Clinic and Dr. Knutson have a financial interest in TapImmune for the triple negative breast cancer treatment.
JACKSONVILLE, Fla., Month Day, 2017 /PRNewswire/ — TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that Peter Hoang, President and Chief Executive Officer, is now available for on-demand viewing at VirtualInvestorConferences.com.
LINK: https://tinyurl.com/1207postpr
TapImmune’s presentation will be available for 90 days. Investors and advisors may download shareholder materials from the “virtual trade booth” for the next three weeks.
Recent Company Highlights
- Potential to be a dominant player in T-cell cancer vaccines by eliciting both helper and killer T-cell responses; durable immune response generated by >90% of vaccinated patients against target
- Evaluating lead candidate in four ongoing Phase 2 studies in ovarian cancer and triple-negative breast cancer; additional studies planned for HER2+ breast cancer vaccine
- Capital efficient clinical strategy: two studies fully funded by grants totaling approx. $17 million
- PolyStart™ antigen expression technology offers multiple out-licensing opportunities
- Highly vetted technology: Collaborations with Mayo Clinic, AstraZeneca, Memorial Sloan Kettering Cancer Center, U.S. Department of Defense
About VirtualInvestorConferences.com
Since 2010, VirtualInvestorConferences.com, created by BetterInvesting (NAIC) and PRNewswire, has been the only monthly virtual investor conference series that provides an interactive forum for presenting companies to meet directly with investors using a graphically-enhanced online platform.
Designed to replicate the look and feel of location-based investor conferences, Virtual Investor Conferences unites PR Newswire’s leading-edge online conferencing and investor communications capabilities with BetterInvesting’s extensive retail investor audience network.
VirtualInvestorconferences.com
John Viglotti
VP, Investor Relations Products and Services
Cision / PR Newswire / MultiVu
350 Hudson Street | 3rd Floor | New York, NY 10014
Phone 201 360 6767 | Mobile 212 729 8350
john.viglotti@nullprnewswire.com
JACKSONVILLE, Florida, December 6, 2017 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced the appointment of Richard Kenney, M.D., F.A.C.P., as Acting Chief Medical Officer. In this newly created role at TapImmune, Dr. Kenney will continue to manage the Company’s ongoing and planned clinical programs for its novel, multi-epitope, T-cell vaccine candidates. Dr. Kenney has served as Head of Clinical Development for TapImmune since May 2017, and served previously as Chief Medical Officer for two biotech publicly traded companies focused on developing immunotherapies for oncology and infectious disease.
“As part of our continuing effort to advance our clinical programs in ovarian and breast cancer as efficiently as possible, we are very pleased to have Dr. Kenney join our executive team to lead the clinical development for TapImmune as Acting Chief Medical Officer,” said TapImmune President and CEO Peter Hoang. “Since joining the Company in February of this year, Dr. Kenney has been successful in driving operational efficiency, working with our clinical teams and investigators to optimize patient enrollment, study design, and execution. We are confident that his continued leadership and dedication will lead to the achievement of many value-driving clinical milestones for TapImmune over the months and years to come.”
Dr. Richard Kenney, Acting Chief Medical Officer of TapImmune, stated, “I believe TapImmune is in a unique position to fulfill important treatment gaps with its novel vaccine technologies, presenting an exciting opportunity for the company and for patients. My primary objective is to continue to optimize the execution of our active Phase 2 clinical trials in ovarian and breast cancer, to launch our planned clinical studies in a timely and cost-effective manner, and to drive TapImmune towards becoming the most efficient clinical organization in our industry. With continued focus on clinical execution, I believe TapImmune can help advance the field of immuno-oncology with its therapeutic vaccine platform and potentially improve the lives of patients who currently have few or no effective treatment options.”
Dr. Kenney also serves as President of ClinReg Biologics, LLC, his own clinical consulting firm. Prior to this role, Dr. Kenney served as Principal Medical Advisor and Chief Medical Officer of Immune Design, where he led the clinical development, pharmacovigilance and regulatory affairs groups to advance the development and commercialization of the company’s prime-boost immunotherapeutic vaccines. Previously, Dr. Kenney served as Chief Medical Officer of Crucell Holland, BV, where he directed the development of a broad platform of vaccines, and as Senior Vice President, Clinical Development for Vical Incorporated, where he led the clinical development of DNA vaccines for cancer immunotherapy and infectious diseases. Dr. Kenney held key positions in vaccine development at GSK Biologicals from 2005 to 2009, most recently as Senior Director of Global Clinical R&D, Vaccines for Viral Diseases. He earned his M.D. degree at Harvard Medical School, then completed his residency in Internal Medicine at Duke University Medical Center and a fellowship in Infectious Diseases at the National Institute of Allergy and Infectious Diseases.
JACKSONVILLE, Fla., December 4, 2017 /PRNewswire/ — TapImmune Inc. (NASDAQ:TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that Peter Hoang, President and Chief Executive Officer, will present live at VirtualInvestorConferences.com on December 7, 2017.
DATE: Thursday, December 7, 2017
TIME: 10:45 AM
LINK: https://tinyurl.com/127prepr
This will be a live, interactive online event where investors are invited to ask the company questions in real-time – both in the presentation hall as well as the association’s “virtual trade booth.” If attendees are not able to join the event live on the day of the conference, an on-demand archive will be available for 90 days.
It is recommended that investors pre-register and run the online system check to save time and receive event updates.
Learn more about the event at www.VirtualInvestorConferences.com.
Recent Company Highlights
- Potential to be a dominant player in T-cell cancer vaccines by eliciting both helper and killer T-cell responses; durable immune response generated by >90% of vaccinated patients against target
- Evaluating lead candidate in four ongoing Phase 2 studies in ovarian cancer and triple-negative breast cancer; additional studies planned for HER2+ breast cancer vaccine
- Capital efficient clinical strategy: two studies fully funded by grants totaling approx. $17 million
- PolyStart™ antigen expression technology offers multiple out-licensing opportunities
- Highly vetted technology: Collaborations with Mayo Clinic, AstraZeneca, Memorial Sloan Kettering Cancer Center, U.S. Department of Defense
About VirtualInvestorConferences.com
Since 2010, VirtualInvestorConferences.com, created by BetterInvesting (NAIC) and PRNewswire, has been the only monthly virtual investor conference series that provides an interactive forum for presenting companies to meet directly with investors using a graphically-enhanced online platform.
Designed to replicate the look and feel of location-based investor conferences, Virtual Investor Conferences unites PR Newswire’s leading-edge online conferencing and investor communications capabilities with BetterInvesting’s extensive retail investor audience network.
VirtualInvestorconferences.com
John Viglotti
VP, Investor Relations Products and Services
Cision / PR Newswire / MultiVu
350 Hudson Street | 3rd Floor | New York, NY 10014
Phone 201 360 6767 | Mobile 212 729 8350
john.viglotti@nullprnewswire.com
JACKSONVILLE, Florida, November 21, 2017 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced its President and CEO, Peter Hoang, will present at the 29th Annual Piper Jaffray Healthcare Conference held November 28-29, 2017 in New York, NY.
TapImmune Event Details:
Date: Wednesday, November 29, 2017
Time: 3:50 p.m. (Eastern Time)
Location: Lotte New York Palace Hotel, New York, NY
An audio webcast will be accessible via the News and Events section of the TapImmune website https://markertherapeutics.com/event/. An archive of the audio will remain available for 90 days following the presentation.
JACKSONVILLE, Florida, November 17, 2017 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced its Chairman and Strategic Advisor Glynn Wilson, Ph.D., and Senior Director of Molecular Biology & Virology Robert Florkiewicz, Ph.D., will give an oral presentation titled, “Increasing the potency of DNA-based immunotherapies using PolyStart™ peptide expression system,” at the World Vaccine & Immunotherapy Congress West Coast, held Thursday, November 30 – December 1, 2017, at the Loews Coronado Bay Resort in San Diego, CA.
The presentation will highlight data on the Company’s proprietary DNA vaccine technology, PolyStart™, and its use in driving DNA vaccine antigen expression and presentation to enable increased visibility of tumor cells to the immune system and more effectively target the cytotoxic activity of CD8+ killer T-cells.
Dr. Wilson and Dr. Florkiewicz will be available for one-on-one meetings at the conference.
TapImmune Event Details:
Title: Increasing the potency of DNA-based immunotherapies using PolyStart™ peptide expression system
Date: Thursday, November 30, 2017
Time: 3:45 p.m. (Pacific Time)
Location: Loews Coronado Bay Resort, San Diego, CA
Recent Corporate and Clinical Developments:
- Appointed Peter Hoang as President and CEO
- Completed enrollment in ongoing Phase 2 dosing study of TPIV200 for treating triple-negative breast cancer (TNBC)
- Began enrolling patients into Phase 2 platinum-sensitive ovarian cancer study under an amended protocol that allows women in their first remission to receive TPIV200 vaccination
- Continued to advance clinical development pipeline in multiple Phase 2 studies in ovarian and breast cancer
Upcoming Anticipated Milestones:
- 2017: Mayo Clinic expected to commence dosing in 280-patient, grant-funded Phase 2 study of TPIV200 in women with advanced TNBC
- 2017: Publish long-term immune response and progression-free survival data from completed Phase 1 clinical study of TPIV200
- 2017: Complete FDA filings required to initiate studies with TPIV 110 for treating HER2/neu+ breast cancer
- 1H 2018: Report interim immune response data from ongoing Phase 2 TNBC
- 1H 2018: Report interim results from ongoing Phase 2 study of TPIV200 in combination with AstraZeneca’s durvalumab in patients with platinum-resistant ovarian cancer
- 1H 2018: Mayo Clinic to initiate Phase 1b/2a study of TPIV100 in women with HER2/neu+ ductal carcinoma in situ (DCIS) breast cancer
JACKSONVILLE, FLORIDA – November 15, 2017 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today provided its business update for the third quarter 2017. A public conference call and live audio webcast is scheduled for today at 4:30 p.m. ET.
“TapImmune continued to advance its clinical and preclinical programs in the third quarter, which I believe positions us very well to achieve a number of important milestones in 2017 and beyond,” said Peter Hoang, President and CEO of TapImmune. “I believe TapImmune is well positioned to advance immuno-oncology with its broadly effective cancer vaccine candidates and proprietary technologies in the indications and treatment settings we are targeting. Having joined the company in late September, I was drawn to TapImmune because I see an opportunity to lead our industry, in translating the promise of cancer immunotherapy into potentially life-changing therapeutic outcomes.
“As we continue to advance our deep clinical pipeline, I am determined to drive TapImmune to its full potential. Our primary objective is to continue executing on our active Phase 2 trials as efficiently as possible. We and our clinical partners also expect to launch additional clinical studies for each of our novel cancer vaccine candidates in order to further highlight their potential and generate even more value for the company. I believe that driving clinical execution should lead to several significant catalysts for the company and its shareholders over the coming quarters and years. I expect to drive efficiencies from an operational, regulatory and commercial standpoint, so that we achieve every milestone we set for the company. In parallel, my goal is to drive enhanced appreciation and support for TapImmune by the investor community. Finally, I intend to pursue opportunities to bolster our pipeline through strategic corporate and business development initiatives, in an effort to further expand the market opportunity for TapImmune.”
Current Clinical Studies:
TPIV200: Lead T-cell vaccine targeting folate receptor alpha
- Multi-center Phase 2 dosing study in triple-negative breast cancer
The randomized study is designed to determine the optimal vaccine dose and regimen that may maximize the anti-tumor immune response in maintenance-phase patients, who have completed standard surgery and chemotherapy/radiation. TapImmune reached its enrollment target ahead of schedule, and currently expects to report interim immune response data in the first half of 2018. - FDA Fast Tracked Phase 2 maintenance therapy study in platinum-sensitive ovarian cancer
TapImmune is currently enrolling women who have completed initial therapy with a platinum regimen and are in first remission. Enrollment remains on track with projections and the company plans to conduct a blinded interim safety and futility analysis once the data from the first half of enrollment is achieved and responses mature, which is currently expected in early 2019. This program benefits from FDA Fast Track and Orphan Drug designation. - Memorial Sloan Kettering-sponsored Phase 2 combination study with AstraZeneca’s durvalumab in platinum-resistant ovarian cancer
Data from the first 27 patients enrolled in the study are currently being analyzed by the study’s clinical investigators at MSKCC. TapImmune anticipates reporting the results based on these 27 patients in the first half of 2018. If the safety profile remains favorable and there are sufficient signs of tumor response, patient enrollment may resume and the study can be completed as designed.
Planned Clinical Studies:
TPIV200:
- Mayo Clinic is expected to commence dosing in a Phase 2 study designed to evaluate the efficacy of TPIV200 in prolonging disease-free survival in women with advanced triple-negative breast cancer. This 280-patient study is planned to commence dosing in the fourth quarter 2017, and is completely funded by a $13.3 million grant from the U.S. Department of Defense.
TPIV100/110 T-cell vaccine targeting HER2/neu:
- TapImmune plans to complete FDA filings required to initiate a Phase 1b/2a clinical study with TPIV110, its five-peptide vaccine candidate for treating women with HER2/neu+ breast cancer.
- Mayo Clinic is expected to initiate a Phase 1b/2a study of TPIV100 in women with an early form of breast cancer called ductal carcinoma in situ (DCIS). This study is also fully funded by a grant from the U.S. Dept. of Defense. If successful, TapImmune’s HER2/neu-targeted vaccine may complement standard surgery and chemotherapy.
Conference Call and Webcast Information:
The company will host a conference call and live audio webcast today, November 15, 2017, at 4:30 p.m. ET. Interested participants and investors may access the conference call by dialing either:
- (855) 238-2333 (U.S.)
- (412) 317-5215 (International)
An audio webcast will be accessible via the New and Events section of the TapImmune website https://markertherapeutics.com/events/. An archive of the webcast and presentation will remain available for 90 days beginning at approximately 6:30 p.m. ET, on November 15, 2017.
TAPIMMUNE INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
TAPIMMUNE INC.
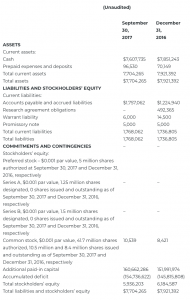
CONDENSED CONSOLIDATED BALANCE SHEETS
TAPIMMUNE INC.
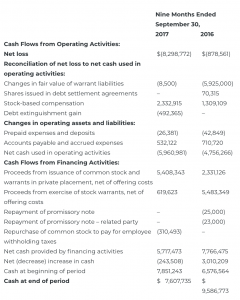
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
JACKSONVILLE, Florida, November 14, 2017 / TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that it has enrolled the final patient in a randomized Phase 2 clinical study of its novel T-cell vaccine candidate TPIV200 for treating triple-negative breast cancer (TNBC). The comprehensive four-arm study is designed to help determine the optimal vaccine dose and regimen to maximize the immune response generated against the vaccine’s molecular target, folate receptor-alpha (FRa), a cancer cell biomarker that is highly correlated with disease recurrence.
Dr. Richard Kenney, Head of Clinical Development for TapImmune, stated, “We are pleased to complete enrollment in this study almost two months ahead of our original projections and want to thank our investigators and their teams for their diligence, as well as their patients for contributing to the development of this vaccine. Our Phase 2 trial focuses on women who have completed initial surgery and radiation/chemotherapy for TNBC at least 60 days prior to randomization. We believe vaccination with TPIV200 during this important window may potentially delay or prevent cancer recurrence by generating robust T-cell immunity against tumor cells. Evaluating multiple vaccine dosing strategies in this Phase 2 trial may enhance our ability to generate optimal immune responses and prevent disease recurrence in future pivotal clinical studies.”
TapImmune and its clinical partners are evaluating TPIV200 in multiple ongoing Phase 2 trials for treating ovarian and breast cancers, including a 280-patient efficacy trial sponsored by the Mayo Clinic that is randomized, double-blind, and placebo-controlled to evaluate disease-free survival in women with advanced TNBC. This larger clinical study to evaluate efficacy is fully funded by a $13.3 million grant from the U.S. Department of Defense and patient dosing is expected to begin by year end 2017.
Peter Hoang, President and CEO of TapImmune, stated, “Triple-negative breast cancer is a difficult-to-treat condition, but one where patients may stand to benefit significantly from immunotherapies that are effective at continually fighting off disease progression long after initial cancer therapy. As a multi-peptide therapeutic vaccine, TPIV200 is designed to do just that. I also want to congratulate our clinical team and thank them for their hard work in getting us to this milestone ahead of schedule. We look forward to reporting interim immunogenicity results in the first half of 2018 and continuing the booster phase of this Phase 2 dosing study, as well as to Mayo Clinic initiating the long-term Phase 2 efficacy study in TNBC.”
JACKSONVILLE, FLORIDA – November 13, 2017 – TapImmune Inc. (NASDAQ: TPIV), a leading clinical-stage immuno-oncology company with ongoing clinical trials in ovarian and breast cancer, today announced that the Company will host a conference call and live audio webcast on Wednesday, November 15, 2017, at 4:30p.m. ET, to provide a corporate and clinical update for the Third quarter 2017.
Reserve Your Seat: Register Today
The event will be webcast live via the Internet on:
November 15, 2017
4:30 p.m. ET
Click Here to register today, or visit https://www.webcaster4.com/Webcast/Page/1651/23491.
Day-of Call-in Information:
To access the live conference call, dial:
- (855) 238-2333 (U.S.)
- (412) 317-5215 (International)
To access the live audio webcast, visit the Events section of the TapImmune website https://markertherapeutics.com/events/. The webcast will also be archived for 90 days beginning at approximately 6:30 p.m. ET, on November 15, 2017.